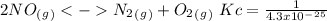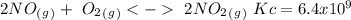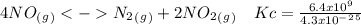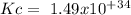Chemistry, 22.10.2019 21:00 kobiemajak
The following reactions have the indicated equilibrium constants at a particular temperature: n2(g) + o2(g) ⇌ 2no(g) kc = 4.3 × 10−25 2no(g) + o2(g) ⇌ 2no2(g) kc = 6.4 × 109 determine the values of the equilibrium constants for the following equations at the same temperature: (a) 4no(g) ⇌ n2(g) + 2no2(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Infants born with severe respiratory problems are sometimes given liquid ventilation: they breathe a liquid that can dissolve more oxygen than air can hold. one of these liquids is a fluorinated compound, cf3(cf2)7br. the solubility of oxygen in this liquid is 66 mlo2 per 100 ml liquid. in contrast, air is 21 % oxygen by volume. calculate the moles of o2 present in an infant's lungs (volume: 12 ml ) if the infant takes a full breath of air. assume a pressure of 1 atm in the lungs.
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3
You know the right answer?
The following reactions have the indicated equilibrium constants at a particular temperature: n2(g)...
Questions

Mathematics, 04.11.2021 18:00


Social Studies, 04.11.2021 18:10

Biology, 04.11.2021 18:10


Mathematics, 04.11.2021 18:10

Biology, 04.11.2021 18:10



Mathematics, 04.11.2021 18:10

Computers and Technology, 04.11.2021 18:10

Physics, 04.11.2021 18:10

Computers and Technology, 04.11.2021 18:10


Biology, 04.11.2021 18:10

Social Studies, 04.11.2021 18:10

Mathematics, 04.11.2021 18:10



Mathematics, 04.11.2021 18:10

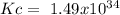
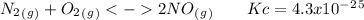
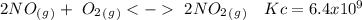
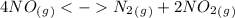
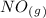 as a reactive in the target reaction and
as a reactive in the target reaction and