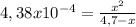For the reaction: ch3nh2(aq) + h2o(aq) ⇌ ch3nh3 +(aq) + oh- determine the change in the ph (δph) for the addition of 6.7 m ch3nh3cl salt to a 4.7 m solution of ch3nh2, kb=4.38 x 10-4 . view available hint(s) for the reaction: ch3nh2(aq) + h2o(aq) ⇌ ch3nh3 +(aq) + oh- determine the change in the ph (δph) for the addition of 6.7 m ch3nh3cl salt to a 4.7 m solution of ch3nh2, kb=4.38 x 10-4 . δph=12.66 δph=1.86 δph=10.49 δph=2.17

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1
You know the right answer?
For the reaction: ch3nh2(aq) + h2o(aq) ⇌ ch3nh3 +(aq) + oh- determine the change in the ph (δph) fo...
Questions

Social Studies, 09.10.2019 12:00

Biology, 09.10.2019 12:00

Social Studies, 09.10.2019 12:00

Mathematics, 09.10.2019 12:00




Mathematics, 09.10.2019 12:00

Mathematics, 09.10.2019 12:00




Computers and Technology, 09.10.2019 12:00

Biology, 09.10.2019 12:00




Mathematics, 09.10.2019 12:00


![kb = \frac{[OH^{-}][CH_{3}NH_{3}^+]}{[CH_{3}NH_{2}]}](/tpl/images/0341/2802/33c02.png) (1)
(1)