Chemistry, 22.10.2019 23:30 hanacat6174
Find δh for the combustion of ethanol (c2h6o) to carbon dioxide and liquid water from the following data. the heat capacity of the bomb calorimeter is 34.65 kj/k and the combustion of 1.752 g of ethanol raises the temperature of the calorimeter from 294.42 k to 295.92 k .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 23.06.2019 18:00
Amolecule is a(n) - a. element that physically combines with another element. b. particle composed of two or more atoms bonded together covalently. c. element that isn't bonded to another element.
Answers: 1

Chemistry, 23.06.2019 18:30
Achemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. the chemical reaction that occurred is shown. na + cl2 ? nacl if the percentage yield of the reaction is 86%, what is the actual yield? show your work, including the use of stoichiometric calculations and conversion factors.
Answers: 1

Chemistry, 23.06.2019 20:00
Asample of water is taken and kept in a beaker in a freezer at a constant temperature of 0°c. if the system is at dynamic equilibrium, which of these statements is true? the rate of freezing is equal to the rate of melting. the rate of freezing is greater than the rate of melting. the amount of ice is greater than the amount of water. the amount of ice is equal to the amount of water.
Answers: 1
You know the right answer?
Find δh for the combustion of ethanol (c2h6o) to carbon dioxide and liquid water from the following...
Questions





Chemistry, 16.07.2019 19:50

English, 16.07.2019 19:50

Spanish, 16.07.2019 19:50


Mathematics, 16.07.2019 19:50


Biology, 16.07.2019 19:50


Mathematics, 16.07.2019 19:50

Mathematics, 16.07.2019 19:50

Business, 16.07.2019 19:50



English, 16.07.2019 19:50

Mathematics, 16.07.2019 19:50

History, 16.07.2019 19:50

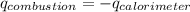
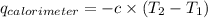
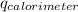 = heat released by calorimeter = ?
= heat released by calorimeter = ?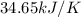
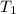 = initial temperature of calorimeter = 294.42 K
= initial temperature of calorimeter = 294.42 K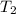 = final temperature of calorimeter = 295.92 K
= final temperature of calorimeter = 295.92 K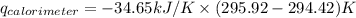
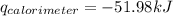
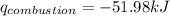
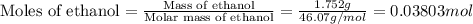
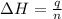
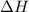 = enthalpy of combustion = ?
= enthalpy of combustion = ?