Two aqueous sulfuric acid solutions containing 20.0 wt% h2so4
(sg = 1.139) and 60.0 wt%...

Two aqueous sulfuric acid solutions containing 20.0 wt% h2so4
(sg = 1.139) and 60.0 wt% h2so4 (sg = 1.498) are mixed to form a
4.00 molar solution (sg = 1.213). taking 100kg of the 20% feed
solution as a basis, calculate the feed ratio (liters 20%
solution/liter 60% solution).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 23.06.2019 14:00
Ahas distinct properties and composition that never vary.
Answers: 1

Chemistry, 23.06.2019 19:40
Aresearcher finds that white bread contains more preservatives and has a higher moisture content than wheat bread. she designs a test to sermons whether the type of bread, white or wheat, affects the amount of mold growth. what must be done for the researcher to accurately measure the dependent variable?
Answers: 1
You know the right answer?
Questions


Mathematics, 15.01.2021 18:20

Mathematics, 15.01.2021 18:20

Business, 15.01.2021 18:20


Business, 15.01.2021 18:20


Mathematics, 15.01.2021 18:20



Mathematics, 15.01.2021 18:20




English, 15.01.2021 18:20


Health, 15.01.2021 18:20

Chemistry, 15.01.2021 18:20

English, 15.01.2021 18:20

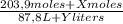 (1)
(1)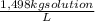 ×
×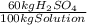 ×
×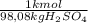 = 0,9164 kmolH₂SO₄ ≡ 916,4 moles
= 0,9164 kmolH₂SO₄ ≡ 916,4 moles


