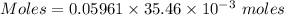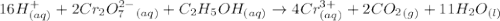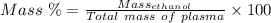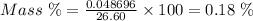Aperson's blood alcohol (c2h5oh) level can be determined by titrating a sample of blood plasma with a potassium dichromate solution. the balanced equation is 16h (aq) 2cr2o72−(aq) c2h5oh(aq) → 4cr3 (aq) 2co2(g) 11h2o(l) if 35.46 ml of 0.05961 m cr2o72− is required to titrate 26.60 g of plasma, what is the mass percent of alcohol in the blood?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
Aperson's blood alcohol (c2h5oh) level can be determined by titrating a sample of blood plasma with...
Questions



Business, 08.06.2021 19:40



Mathematics, 08.06.2021 19:40

Mathematics, 08.06.2021 19:40

Mathematics, 08.06.2021 19:40





Computers and Technology, 08.06.2021 19:40


Geography, 08.06.2021 19:40

Mathematics, 08.06.2021 19:40




Mathematics, 08.06.2021 19:40

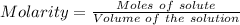
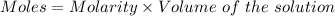
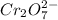 :
: