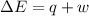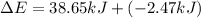Chemistry, 23.10.2019 00:30 kandigirl9990
Limestone stalactites and stalagmites are formed in caves by the following reaction: ca2 (aq) 2hco−3(aq)→caco3(s) co2(g) h2o(l) if 1 mol of caco3 forms at 298 k under 1 atm pressure, the reaction performs 2.47 kj of p−v work, pushing back the atmosphere as the gaseous co2 forms. at the same time, 38.65 kj of heat is absorbed from the environment. what is the value of δe for this reaction

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
Limestone stalactites and stalagmites are formed in caves by the following reaction: ca2 (aq) 2hco−...
Questions








Biology, 17.12.2019 19:31

Biology, 17.12.2019 19:31


Mathematics, 17.12.2019 19:31

History, 17.12.2019 19:31


Health, 17.12.2019 19:31



Advanced Placement (AP), 17.12.2019 19:31


History, 17.12.2019 19:31

 for this reaction is 36.18 kJ
for this reaction is 36.18 kJ