
Chemistry, 23.10.2019 00:50 Drellibus70011
Nthe formation of chloromethane and hydrogen chloride. the overall reaction is 2ch4(g)+3cl2(g)⟶2ch3cl(g)+2hcl(g)+2 cl−(g) 2ch4(g)+3cl2(g)⟶2ch3cl(g)+2hcl(g)+2 cl−(g) suppose that a chemist combines 295 ml295 ml of methane and 725 ml725 ml of chlorine at stp in a 2.00 l2.00 l flask. the flask is then allowed to stand at 298 k.298 k. if the reaction reaches 77.0%77.0% completion, what is the total pressure in the flask?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
You know the right answer?
Nthe formation of chloromethane and hydrogen chloride. the overall reaction is 2ch4(g)+3cl2(g)⟶2ch3c...
Questions


Chemistry, 31.08.2020 04:01

Mathematics, 31.08.2020 04:01


History, 31.08.2020 04:01

Mathematics, 31.08.2020 04:01

History, 31.08.2020 04:01

Mathematics, 31.08.2020 04:01

Mathematics, 31.08.2020 04:01



Mathematics, 31.08.2020 04:01

Mathematics, 31.08.2020 04:01

Computers and Technology, 31.08.2020 04:01


Physics, 31.08.2020 04:01

Chemistry, 31.08.2020 04:01

History, 31.08.2020 04:01

Mathematics, 31.08.2020 04:01

History, 31.08.2020 04:01

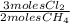 = 0,0198 moles Cl₂. That means that 0,0324-0,0198 = 0,0126 moles of Cl₂ are in excess.
= 0,0198 moles Cl₂. That means that 0,0324-0,0198 = 0,0126 moles of Cl₂ are in excess.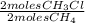 = 0,0102 moles of CH₃Cl -that are the same moles of HCl and Cl⁻-
= 0,0102 moles of CH₃Cl -that are the same moles of HCl and Cl⁻-

