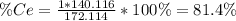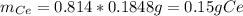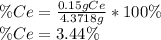Chemistry, 23.10.2019 16:50 chelseychew32
To find the ce4+ content in a solid sample, 4.3718 g of the solid sample were dissolved and treated with excess iodate to precipitate the ce4+ as ce(io3)4. the precipitate was collected, washed well, dried, and ignited to produce 0.1848 g of ceo2 (fm 172.114). what was the weight percentage of ce (am 140.116) in the original sample?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3
You know the right answer?
To find the ce4+ content in a solid sample, 4.3718 g of the solid sample were dissolved and treated...
Questions

Arts, 19.01.2021 01:00

Mathematics, 19.01.2021 01:00

Mathematics, 19.01.2021 01:00

Chemistry, 19.01.2021 01:00

Mathematics, 19.01.2021 01:00


Health, 19.01.2021 01:00

Mathematics, 19.01.2021 01:00

English, 19.01.2021 01:00


Advanced Placement (AP), 19.01.2021 01:00



Mathematics, 19.01.2021 01:00

Biology, 19.01.2021 01:00

English, 19.01.2021 01:00


Mathematics, 19.01.2021 01:00


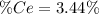
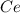 into the
into the 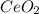 by using their respective molar masses as shown below:
by using their respective molar masses as shown below: