
Chemistry, 16.09.2019 05:50 Bleejones00
Write a balanced chemical equation for the reaction of zncl2 with excess naoh to produce na2zn(oh)4 sodium zincate. what mass of sodium zincate can be produced from 2.00 g of zncl2 with excess naoh by this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1
You know the right answer?
Write a balanced chemical equation for the reaction of zncl2 with excess naoh to produce na2zn(oh)4...
Questions

Computers and Technology, 21.09.2020 02:01



Biology, 21.09.2020 02:01

History, 21.09.2020 02:01





Mathematics, 21.09.2020 02:01




Mathematics, 21.09.2020 02:01


Mathematics, 21.09.2020 02:01

Mathematics, 21.09.2020 02:01



Mathematics, 21.09.2020 02:01

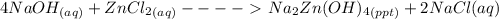
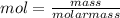
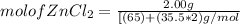
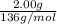
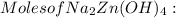
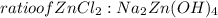
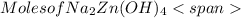 = 0.0147 mol
= 0.0147 mol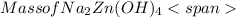 = [(23 * 2)+(65)+(16 * 4) + (1*4)] g/mol * 0.0147 mol
= [(23 * 2)+(65)+(16 * 4) + (1*4)] g/mol * 0.0147 mol

