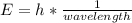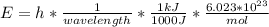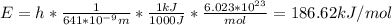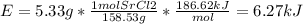Chemistry, 23.10.2019 17:00 camirialchambers17
In fireworks, the heat of the reaction of an oxidizing agent, such as kclo4, with an organic compound excites certain salts, which emit specific colors. strontium salts have an intense emission at 641 nm. what is the energy (in kj) of this emission for 5.33 g of the chloride salt of strontium? assume that all the heat produced is converted to emitted light. enter to 2 decimal places.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
In fireworks, the heat of the reaction of an oxidizing agent, such as kclo4, with an organic compoun...
Questions


English, 21.11.2020 01:00


Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Social Studies, 21.11.2020 01:00


Spanish, 21.11.2020 01:00


Mathematics, 21.11.2020 01:00

Health, 21.11.2020 01:00


Mathematics, 21.11.2020 01:00



Arts, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00