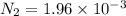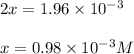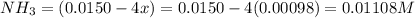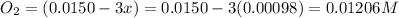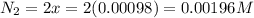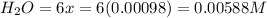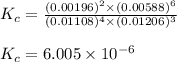Chemistry, 23.10.2019 17:00 tasnimabdallah971
Be sure to answer all parts. the first step in hno3 production is the catalyzed oxidation of nh3. without a catalyst, a different reaction predominates: 4 nh3(g) +3 o2 (g) ⇌ 2 n2(g) + 6 h2o(g) when 0.0150 mol of nh3(g) and 0.0150 mol of o2(g) are placed in a 1.00−l container at a certain temperature, the n2 concentration at equilibrium is 1.96 × 10−3 m. calculate kc.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1
You know the right answer?
Be sure to answer all parts. the first step in hno3 production is the catalyzed oxidation of nh3. wi...
Questions



Geography, 24.04.2021 21:00

Mathematics, 24.04.2021 21:00

Biology, 24.04.2021 21:00



Computers and Technology, 24.04.2021 21:00

English, 24.04.2021 21:00

Computers and Technology, 24.04.2021 21:00



English, 24.04.2021 21:00


History, 24.04.2021 21:00

English, 24.04.2021 21:00

Social Studies, 24.04.2021 21:00


Mathematics, 24.04.2021 21:00

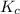 for the reaction is
for the reaction is 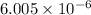
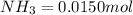
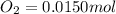
![[NH_3]_i=\frac{0.0150}{1.00}=0.0150M](/tpl/images/0342/7531/438ea.png)
![[O_2]_i=\frac{0.0150}{1.00}=0.0150M](/tpl/images/0342/7531/0aac8.png)
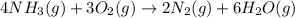
![K_c=\frac{[N_2]^2[H_2O]^6}{[NH_3]^4[O_2]^3}](/tpl/images/0342/7531/b62d0.png) .......(1)
.......(1)