Chemistry, 23.10.2019 18:30 dannyelleparker9680
Atmospheric pressure at sea level is equal to a column of 760 mm hg. oxygen makes up 21 percent of the atmosphere by volume. the partial pressure of oxygen (po2) in such conditions is atmospheric pressure at sea level is equal to a column of 760 mm hg. oxygen makes up 21 percent of the atmosphere by volume. the partial pressure of oxygen (po2) in such conditions is 21/760 16 mm hg 760/21 120/75 160 mm hg submitr

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

You know the right answer?
Atmospheric pressure at sea level is equal to a column of 760 mm hg. oxygen makes up 21 percent of t...
Questions


History, 29.07.2019 07:30

Mathematics, 29.07.2019 07:30





Mathematics, 29.07.2019 07:30

Mathematics, 29.07.2019 07:30





Mathematics, 29.07.2019 07:30


English, 29.07.2019 07:30


Biology, 29.07.2019 07:30


Social Studies, 29.07.2019 07:30

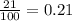
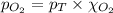
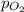 = partial pressure of oxygen = ?
= partial pressure of oxygen = ?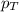 = total pressure of air = 760 mmHg
= total pressure of air = 760 mmHg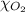 = mole fraction of oxygen = 0.21
= mole fraction of oxygen = 0.21