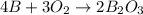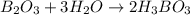Chemistry, 23.10.2019 18:30 jasmin2344
When elemental boron, b, is burned in oxygen gas, the product is diboron trioxide. if the diboron trioxide is then reacted with a measured quantity of water, it reacts with the water to form what is commonly known as boric acid, b(oh)3. write a balanced chemical equation for each of these processes. (use the lowest possible coefficients. omit states-of-matter in your answer.)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2


Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
You know the right answer?
When elemental boron, b, is burned in oxygen gas, the product is diboron trioxide. if the diboron tr...
Questions

Computers and Technology, 12.02.2021 04:00



Mathematics, 12.02.2021 04:00


English, 12.02.2021 04:00

Mathematics, 12.02.2021 04:00


Mathematics, 12.02.2021 04:00


History, 12.02.2021 04:00


Mathematics, 12.02.2021 04:00

English, 12.02.2021 04:00

Geography, 12.02.2021 04:00


Computers and Technology, 12.02.2021 04:00

French, 12.02.2021 04:00