
Chemistry, 23.10.2019 18:00 krishawnnn
For the generic equilibrium ha(aq) ⇌ h+(aq) + a−(aq), which of these statements is true? for the generic equilibrium , which of these statements is true? if you add the soluble salt ka to a solution of ha that is at equilibrium, the concentration of ha would decrease. if you add the soluble salt ka to a solution of ha that is at equilibrium, the ph would increase. the equilibrium constant for this reaction changes as the ph changes. if you add the soluble salt ka to a solution of ha that is at equilibrium, the concentration of a− would decrease.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

You know the right answer?
For the generic equilibrium ha(aq) ⇌ h+(aq) + a−(aq), which of these statements is true? for the ge...
Questions



History, 12.03.2020 18:49

Mathematics, 12.03.2020 18:49

Computers and Technology, 12.03.2020 18:49







Social Studies, 12.03.2020 18:50








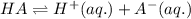
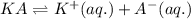
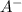 ion is getting increased on the product side, so the equilibrium will shift in the direction to minimize this effect, which is in the direction of HA.
ion is getting increased on the product side, so the equilibrium will shift in the direction to minimize this effect, which is in the direction of HA.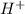 ions are getting decreases. This will increase the pH of the solution.
ions are getting decreases. This will increase the pH of the solution.

