
He chlorination of methane occurs in a number of steps that results in the formation of chloromethane and hydrogen chloride. the overall reaction is 2ch4(g)+3cl2(g)⟶2ch3cl(g)+2hcl(g)+2 cl−(g) 2ch4(g)+3cl2(g)⟶2ch3cl(g)+2hcl(g)+2 cl−(g) suppose that a chemist combines 295 ml295 ml of methane and 725 ml725 ml of chlorine at stp in a 2.00 l2.00 l flask. the flask is then allowed to stand at 298 k.298 k. if the reaction reaches 77.0%77.0% completion, what is the total pressure in the flask?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
He chlorination of methane occurs in a number of steps that results in the formation of chloromethan...
Questions

Mathematics, 27.10.2019 23:43




Spanish, 27.10.2019 23:43

History, 27.10.2019 23:43


English, 28.10.2019 00:43

Computers and Technology, 28.10.2019 00:43


Mathematics, 28.10.2019 00:43


Mathematics, 28.10.2019 00:43

Computers and Technology, 28.10.2019 00:43

Mathematics, 28.10.2019 00:43


English, 28.10.2019 00:43


Mathematics, 28.10.2019 00:43

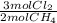 = 0,0198 moles Cl₂. That means that 0,0324-0,0198 = -0,0126 moles of Cl₂ are in excess-.
= 0,0198 moles Cl₂. That means that 0,0324-0,0198 = -0,0126 moles of Cl₂ are in excess-.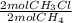 = 0,0102 moles of CH₃Cl -that are the same moles of HCl and Cl⁻-
= 0,0102 moles of CH₃Cl -that are the same moles of HCl and Cl⁻-

