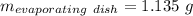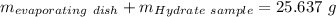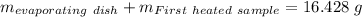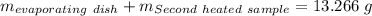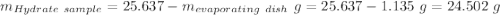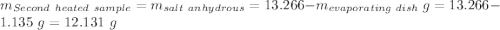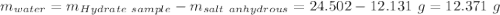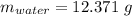Astudent heats a sample of hydrate once, and the mass of the sample and the evaporating dish is 16.428 g. after a second heating cycle, the mass of the sample and the dish is 13.266 g. the student stops the heat/cool/weigh cycle after the second time. if the original mass of the evaporating dish and the hydrate was 25.637 g, and the mass of the evaporating dish alone was 1.135 g, what is the mass of water removed from the sample by heating? provide your response to three digits after the decimal.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
Astudent heats a sample of hydrate once, and the mass of the sample and the evaporating dish is 16.4...
Questions



Social Studies, 01.12.2020 06:20

Mathematics, 01.12.2020 06:20


Mathematics, 01.12.2020 06:20

Advanced Placement (AP), 01.12.2020 06:20


Mathematics, 01.12.2020 06:20





English, 01.12.2020 06:20

Biology, 01.12.2020 06:20


History, 01.12.2020 06:20

Advanced Placement (AP), 01.12.2020 06:20

Biology, 01.12.2020 06:20

Mathematics, 01.12.2020 06:20