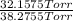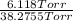Chemistry, 23.10.2019 19:20 SavgeKid7578
Adilute solution of bromine in carbon tetrachloride behaves as an ideal-dilute solution. the vapour pressure of pure ccl4 is 33.85 torr at 298k. the henry’s law constant when the concentration of br2 is expressed as a mole fraction is 122.36 torr. calculate the vapour pressure of each component, the total pressure, and the composition of the vapour phase when the mole fraction of br2 is 0.050, on the assumption that the conditions of the ideal-dilute solution are satisfied at this condition

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1


Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
Adilute solution of bromine in carbon tetrachloride behaves as an ideal-dilute solution. the vapour...
Questions


English, 27.09.2020 14:01

Spanish, 27.09.2020 14:01





Chemistry, 27.09.2020 14:01

Mathematics, 27.09.2020 14:01


Chemistry, 27.09.2020 14:01


English, 27.09.2020 14:01




Mathematics, 27.09.2020 14:01



History, 27.09.2020 14:01

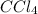 = 33.85 Torr
= 33.85 Torr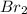 = 122.36 torr
= 122.36 torr