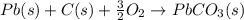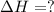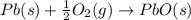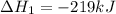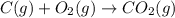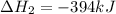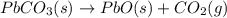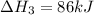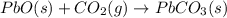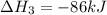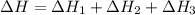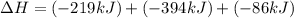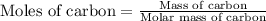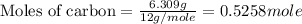Chemistry, 23.10.2019 19:30 desiiraee6265
Use the following information to calculate the amount of heat involved in the complete reaction of 6.309 of carbon to from pbco3 (s) in reaction 4. be sure to give the proper sign (positive or negative) with your answer. (1) pb(s)+1/2 o2 right arrow pbo(s) (2) c(g)+o2(g) right arrow co2(g) (3) pbco3(s)right arrow pbo(s)+co2(g) (4) pb(s)+c(s)+3/2 o2(g) right arrow pbo3(s) delta h degree rsn= -219 kj delta h degree rsn= -394 kj delta h degree rsn= 86 kj

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2
You know the right answer?
Use the following information to calculate the amount of heat involved in the complete reaction of 6...
Questions


History, 02.07.2019 05:00

Biology, 02.07.2019 05:00

Mathematics, 02.07.2019 05:00

Mathematics, 02.07.2019 05:00


History, 02.07.2019 05:00


Mathematics, 02.07.2019 05:00


Advanced Placement (AP), 02.07.2019 05:00


Mathematics, 02.07.2019 05:00

Mathematics, 02.07.2019 05:00

Biology, 02.07.2019 05:00

Biology, 02.07.2019 05:00


Biology, 02.07.2019 05:00