
Chemistry, 23.10.2019 20:00 hetdashadia123
Ethanol, c2h5oh, is produced industrially from ethylene, c2h4, by the following sequence of reactions: 3c2h4 + 2h2so4 → c2h5hso4 + (c2h5)2so4 c2h5hso4 + (c2h5)2so4 + 3h2o → 3c2h5oh + 2h2so4 what volume of ethylene at stp is required to produce 1.000 metric ton (1000 kg) of ethanol if the overall yield of ethanol is 90.1%

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3

Chemistry, 23.06.2019 06:00
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1
You know the right answer?
Ethanol, c2h5oh, is produced industrially from ethylene, c2h4, by the following sequence of reaction...
Questions



Spanish, 13.07.2019 13:30

History, 13.07.2019 13:30

Mathematics, 13.07.2019 13:30

Mathematics, 13.07.2019 13:30

Social Studies, 13.07.2019 13:30



Mathematics, 13.07.2019 13:30

Physics, 13.07.2019 13:30


History, 13.07.2019 13:30


History, 13.07.2019 13:30



Mathematics, 13.07.2019 13:30


Physics, 13.07.2019 13:30

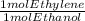 * 100/90.1 = 2.4128* 10⁴ mol ethylene
* 100/90.1 = 2.4128* 10⁴ mol ethylene

