
If 8.0 g of nh4hs(s)nh4hs(s) is placed in a sealed vessel with a volume of 1.0 l and heated to 200°c200 °c the reaction nh4hs(s)⥫⥬==nh3(g)+h2s(g)nh4hs(s) ⇌ nh3(g)+h2s(g) will occur. when the system comes to equilibrium, some nh4hs(s)nh4hs(s) is still present. which of the following changes will lead to a reduction in the amount of nh4hs(s)nh4hs(s) that is present, assuming in all cases that equilibrium is re-established following the change? adding more nh3(g)nh3(g) to the vessel adding more h2s(g)h2s(g) to the vessel adding more nh4hs(s)nh4hs(s) to the vessel increasing the volume of the vessel decreasing the volume of the vessel

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
If 8.0 g of nh4hs(s)nh4hs(s) is placed in a sealed vessel with a volume of 1.0 l and heated to 200°c...
Questions





Computers and Technology, 14.07.2020 01:01





History, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01


Mathematics, 14.07.2020 01:01


Mathematics, 14.07.2020 01:01


Mathematics, 14.07.2020 01:01


English, 14.07.2020 01:01

English, 14.07.2020 01:01

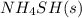 to the vessel and increasing the volume of the vessel
to the vessel and increasing the volume of the vessel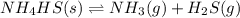
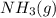 to the vessel
to the vessel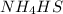 will increase.
will increase.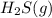 to the vessel
to the vessel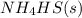 to the vessel
to the vessel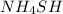 is added, the concentration of reactants get increased. So, by Le-Chatelier's principle, the equilibrium will shift in the direction where concentration of reactants must decrease, which is in the forward direction.
is added, the concentration of reactants get increased. So, by Le-Chatelier's principle, the equilibrium will shift in the direction where concentration of reactants must decrease, which is in the forward direction.

