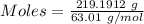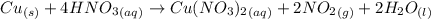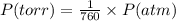Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and photochemical smog. what volume of nitrogen dioxide is formed at 724 torr and 28.2° c by reacting 4.84 cm3 of copper (d = 8.95 g/cm3) with 227 ml of nitric acid (d = 1.42 g/cm3, 68.0% hno3 by mass)? cu + 4hno3 = cu(no3)2 + 2no2 + 2h2o

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and ph...
Questions

Biology, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00



Mathematics, 04.12.2020 14:00



Mathematics, 04.12.2020 14:00

History, 04.12.2020 14:00

Health, 04.12.2020 14:00






Social Studies, 04.12.2020 14:00

English, 04.12.2020 14:00


English, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00

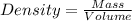
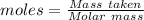
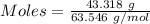
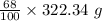 = 219.1912 g
= 219.1912 g