
Chemistry, 23.10.2019 23:00 robbinsjeffrey271
Asample of nitrosyl bromide (nobr) decomposes according to the equation 2nobr(g)⇌2no(g)+br2(g) an equilibrium mixture in a 5.00-l vessel at 100 ∘c contains 3.29 g of nobr, 3.04 g of no, and 8.10 g of br2. part apart complete calculate kc. express your answer using three significant figures. 0.116 previous answers correct part bpart complete what is the total pressure exerted by the mixture of gases? express your answer to three significant figures and include the appropriate units. 1.11 atm previous answers correct part c what was the mass of the original sample of nobr?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2
You know the right answer?
Asample of nitrosyl bromide (nobr) decomposes according to the equation 2nobr(g)⇌2no(g)+br2(g) an eq...
Questions

Arts, 01.03.2021 21:50


English, 01.03.2021 21:50


Mathematics, 01.03.2021 21:50

Mathematics, 01.03.2021 21:50



Mathematics, 01.03.2021 21:50






History, 01.03.2021 21:50

Biology, 01.03.2021 21:50

Mathematics, 01.03.2021 21:50


Mathematics, 01.03.2021 21:50

Biology, 01.03.2021 21:50

![kc = \frac{[Br_{2}][NO]^2{[NOBr]^2}](/tpl/images/0343/4270/3b92b.png) (1)
(1)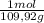 = 0,0299 moles/ 5,00L = 5,986x10⁻³M
= 0,0299 moles/ 5,00L = 5,986x10⁻³M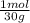 = 0,101 moles/ 5,00L = 0,0203M
= 0,101 moles/ 5,00L = 0,0203M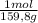 = 0,0507 moles/ 5,00L = 0,0101M
= 0,0507 moles/ 5,00L = 0,0101M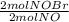 = 0,101 mol NOBr
= 0,101 mol NOBr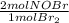 = 0,101 mol NOBr
= 0,101 mol NOBr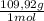 = 25,5 g of NOBr
= 25,5 g of NOBr

