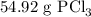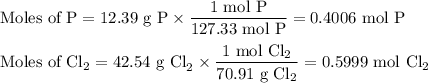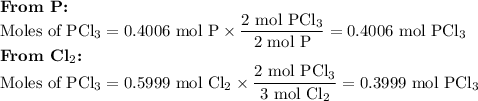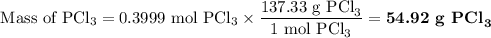Chemistry, 24.10.2019 01:00 peggycab4201
Phosphorous trichloride can be formed via a combination reaction from its elements. phosphorous is often represented by its empirical formula, p, in chemical equations, as is carbon. if 12.39 g of phosphorous is combined with 42.54 g of chlorine, what mass of phosphorous trichloride could be formed?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1


Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
You know the right answer?
Phosphorous trichloride can be formed via a combination reaction from its elements. phosphorous is o...
Questions


Mathematics, 11.10.2020 16:01

English, 11.10.2020 16:01




Mathematics, 11.10.2020 16:01

Chemistry, 11.10.2020 16:01


World Languages, 11.10.2020 16:01

Mathematics, 11.10.2020 16:01

Mathematics, 11.10.2020 16:01

Geography, 11.10.2020 16:01



Mathematics, 11.10.2020 16:01




English, 11.10.2020 16:01