
Chemistry, 24.10.2019 03:00 morganlynn18
You have prepared a buffer by combining 5.00 ml 0.010 m ha (weak acid) and 3.00 ml 0.010 m naa (provides a -, the conjugate base of ha). pk a for ha is 6.23. how many moles of a - will be present in the mixture after addition of 0.20 ml 0.010 m naoh? (suggestion: start by calculating the moles of everything in the mixture.) enter the numerical value, without units, to at least 2 significant figures. you may use e-format scientific notation, e. g., 1.23x10 -4 would be entered as 1.23e-4. (note that the acid and base do not add up to 20.00 ml -- water must be added. that does not affect how you answer the question.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

You know the right answer?
You have prepared a buffer by combining 5.00 ml 0.010 m ha (weak acid) and 3.00 ml 0.010 m naa (prov...
Questions






Chemistry, 19.12.2019 05:31



Biology, 19.12.2019 05:31


History, 19.12.2019 05:31


Mathematics, 19.12.2019 05:31

Geography, 19.12.2019 05:31


Mathematics, 19.12.2019 05:31

English, 19.12.2019 05:31



Mathematics, 19.12.2019 05:31

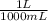 = 5x10⁻³ L of HA
= 5x10⁻³ L of HA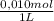 = 5x10⁻⁵ mol of HA
= 5x10⁻⁵ mol of HA

