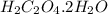Chemistry, 24.10.2019 03:00 austinmontgomep7foxp
Barium oxalate is used as a colorant to produce the green color in fireworks. imagine that you have been assigned to prepare barium oxalate by reacting barium hydroxide octahydrate (ba(oh)2·8h2o) with oxalic acid dihydrate (h2c2o4·2h2o). find the number of grams of oxalic acid dihydrate that would be required to completely react with 5.3 g of barium hydroxide octahydrate. use correct significant figures. do not include a unit with your answer or it will be counted wrong.
h2c2o4·2h2o 126.07 g/mol
h2c2o4 90.03 g/mol
ba(oh)2·8h2o 315.46 g/mol
ba(oh)2 171.34 g/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1
You know the right answer?
Barium oxalate is used as a colorant to produce the green color in fireworks. imagine that you have...
Questions



Mathematics, 25.11.2020 14:00

Mathematics, 25.11.2020 14:00






Computers and Technology, 25.11.2020 14:00

Advanced Placement (AP), 25.11.2020 14:00







Computers and Technology, 25.11.2020 14:00


Spanish, 25.11.2020 14:00