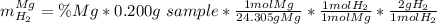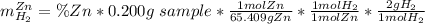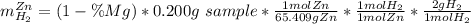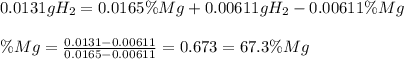Chemistry, 24.10.2019 04:00 sierravick123owr441
A0.200 g sample of magnesium and zinc is placed in a rigid 1 l vessel containing dry air at 25 °c and 1 atm. the magnesium and zinc are treated with dilute sulfuric acid to produce hydrogen gas according to the following balanced reactions: mg (s) + h2so4 (aq) → mg2+ (aq) + so42– (aq) + h2 (g) zn (s) + h2so4 (aq) → zn2+ (aq) + so42– (aq) + h2 (g) the hydrogen gas is then completely combusted in the same vessel to form water as a product. the vessel is then cooled back to 25 °c (assume that all the water condenses) and the pressure is found to be 0.95 atm. what is the mass percentage of magnesium in the mixture?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2
You know the right answer?
A0.200 g sample of magnesium and zinc is placed in a rigid 1 l vessel containing dry air at 25 °c an...
Questions


Mathematics, 04.02.2021 19:20

Mathematics, 04.02.2021 19:20


Mathematics, 04.02.2021 19:20



English, 04.02.2021 19:20

Mathematics, 04.02.2021 19:20

Mathematics, 04.02.2021 19:20





Mathematics, 04.02.2021 19:20




Chemistry, 04.02.2021 19:20

Mathematics, 04.02.2021 19:20

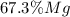
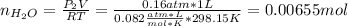
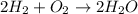
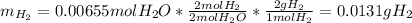
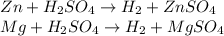
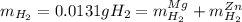
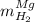 accounts for the hydrogen yielded by the magnesium and
accounts for the hydrogen yielded by the magnesium and 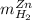 by the zinc which are computed in terms of the stoichiometry and the initial sample's composition as shown below:
by the zinc which are computed in terms of the stoichiometry and the initial sample's composition as shown below: