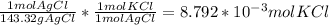Chemistry, 25.10.2019 00:43 cookiebrain72
Consider the following balanced chemical equation, kcl(aq) agno3(aq) → agcl(s) kno3(aq)when a sample of impure potassium chloride (0.900 g) was dissolved in water, and treated with excess silver nitrate (agno3), 1.26 g of silver chloride (agcl) was precipitated. calculate the percentage kcl in the original sample.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

You know the right answer?
Consider the following balanced chemical equation, kcl(aq) agno3(aq) → agcl(s) kno3(aq)when a sample...
Questions

Mathematics, 05.10.2019 22:30

Biology, 05.10.2019 22:30

Mathematics, 05.10.2019 22:30

Biology, 05.10.2019 22:30


Physics, 05.10.2019 22:30


Mathematics, 05.10.2019 22:30


Mathematics, 05.10.2019 22:30


History, 05.10.2019 22:30





Chemistry, 05.10.2019 22:30

Mathematics, 05.10.2019 22:30


Mathematics, 05.10.2019 22:30