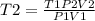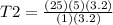Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
You know the right answer?
Calculate the final temperature (°c) of a gas if 32.0 l of the gas at 25°c and 1.00 atm is compresse...
Questions

Mathematics, 23.04.2021 19:30


Arts, 23.04.2021 19:30


Mathematics, 23.04.2021 19:30




Health, 23.04.2021 19:30

Mathematics, 23.04.2021 19:30




Mathematics, 23.04.2021 19:30

Mathematics, 23.04.2021 19:30



Mathematics, 23.04.2021 19:30

Mathematics, 23.04.2021 19:30