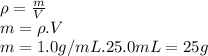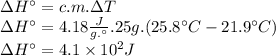Chemistry, 25.10.2019 02:43 hayleymckee
Instant cold packs, often used to ice athletic injuries on the field, contain ammonium nitrate and water separated by a thin plastic divider. when the divider is broken, the ammonium nitrate dissolves according to the following endothermic reaction: nh4no3(> nh4 ^+(aq) + no3^-(aq). in order to measure the enthalpy change for this reaction, 1.25 g of nh4no3 is dissolved in enough water to make 25.0 ml of solution. the initial temperature is 25.8 degrees c and the final temperature (after the solid dissolves) is 21.9 degrees c. calculate the change in enthalpy for the reaction. (use 1.0g/ml as the density of the solution and 4.18 j/g . degrees c as the specific heat capacity.)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
Instant cold packs, often used to ice athletic injuries on the field, contain ammonium nitrate and w...
Questions

Mathematics, 19.05.2021 17:30

Mathematics, 19.05.2021 17:30


Mathematics, 19.05.2021 17:30


Mathematics, 19.05.2021 17:30



Biology, 19.05.2021 17:30


Advanced Placement (AP), 19.05.2021 17:30

English, 19.05.2021 17:30

Mathematics, 19.05.2021 17:30

Mathematics, 19.05.2021 17:30

Mathematics, 19.05.2021 17:30





Mathematics, 19.05.2021 17:30