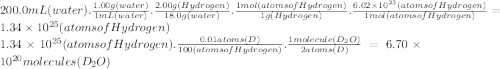Chemistry, 25.10.2019 19:43 sciencefanfae7248
The natural abundances of the two stable isotopes of hydrogen (hydrogen and deuterium) are 99.99 percent and 0.01 percent. assume that water exists as either _1^1 h_2o or _1^2 d_2o. calculate the number of d_2o molecules in 200.0 ml of water (density = 1.00 g/ml). enter your answer in scientific notation.

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
The natural abundances of the two stable isotopes of hydrogen (hydrogen and deuterium) are 99.99 per...
Questions




Mathematics, 07.10.2019 13:00



Mathematics, 07.10.2019 13:00

Mathematics, 07.10.2019 13:00


Chemistry, 07.10.2019 13:00

English, 07.10.2019 13:00


Mathematics, 07.10.2019 13:00




Biology, 07.10.2019 13:00