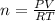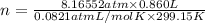Chemistry, 25.10.2019 19:43 nschavez123
Olympic cyclists fill their tires with helium to make them lighter. assume that the volume of the tire is 860 ml , that it is filled to a total pressure of 120 psi , and that the temperature is 26 ∘c. also, assume an average molar mass for air of 28.8 g/mol.
a) calculate the mass of air in an air filled tire.
b) calculate the mas of helium in a helium-filled tire.
c) what is the mass difference between the two?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Why should the scientific method be used to answer a question? a. it provides a way to test an idea without any bias. b. it provides a way to test a hypothesis. c. it provides a way to ensure all hypotheses are proven correct. d. it provides a way to quickly turn a hypothesis into a scientific theory.
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
Olympic cyclists fill their tires with helium to make them lighter. assume that the volume of the ti...
Questions


Computers and Technology, 29.03.2022 01:00

Mathematics, 29.03.2022 01:00


Mathematics, 29.03.2022 01:00

Mathematics, 29.03.2022 01:10

Biology, 29.03.2022 01:20


Arts, 29.03.2022 01:30


Mathematics, 29.03.2022 01:30


Computers and Technology, 29.03.2022 01:40


Mathematics, 29.03.2022 01:40

Social Studies, 29.03.2022 01:40