
Chemistry, 25.10.2019 22:43 sarahhans6711
In the reaction of aluminum and iron(iii) oxide to form iron and aluminum oxide, δh is -850 kj. how many grams of aluminum oxide are formed when 350 kj of heat are released? 2 al (s) + fe2o3 (s) → 2 fe (s) + al2o3 (s) δh= -850 kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
In the reaction of aluminum and iron(iii) oxide to form iron and aluminum oxide, δh is -850 kj. how...
Questions










Mathematics, 27.11.2019 20:31



Mathematics, 27.11.2019 20:31




Advanced Placement (AP), 27.11.2019 20:31

Mathematics, 27.11.2019 20:31


History, 27.11.2019 20:31

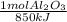 = 0,412 moles of Aluminium oxide.
= 0,412 moles of Aluminium oxide.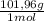 = 41,98 g
= 41,98 g

