
Chemistry, 25.10.2019 23:43 saabrrinnaaa
At 650 k, the reaction mgco3(s)⇌mgo(s)+co2(g)mgco3(s)⇌mgo( s)+co2(g) has kp=0.026kp=0.026. a 10.0-l container at 650 k has 1.0 g of mgo(s) and co2co2 at p=0.0260 atmp=0.0260 atm. the container is then compressed to a volume of 0.100 l. find the mass of mgco3mgco3 that is formed.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
You know the right answer?
At 650 k, the reaction mgco3(s)⇌mgo(s)+co2(g)mgco3(s)⇌mgo( s)+co2(g) has kp=0.026kp=0.026. a 10.0-l...
Questions



Biology, 15.04.2021 19:30






Social Studies, 15.04.2021 19:30

Mathematics, 15.04.2021 19:30







English, 15.04.2021 19:30



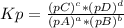 , where pX is the pressure of X in equilibrium.
, where pX is the pressure of X in equilibrium. 

