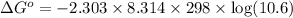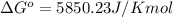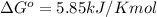Chemistry, 26.10.2019 00:43 noellelovebug1214
At 25 ∘c , the equilibrium partial pressures for the reaction were found to be pa=5.16 bar, pb=5.04 bar, pc=4.11 bar, and pd=4.85 bar . a(g)+2b(g)↽−−⇀4c(g)+d(g) what is the standard change in gibbs free energy of this reaction at 25 ∘c ? δ∘rxn= kjmol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Infants born with severe respiratory problems are sometimes given liquid ventilation: they breathe a liquid that can dissolve more oxygen than air can hold. one of these liquids is a fluorinated compound, cf3(cf2)7br. the solubility of oxygen in this liquid is 66 mlo2 per 100 ml liquid. in contrast, air is 21 % oxygen by volume. calculate the moles of o2 present in an infant's lungs (volume: 12 ml ) if the infant takes a full breath of air. assume a pressure of 1 atm in the lungs.
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
At 25 ∘c , the equilibrium partial pressures for the reaction were found to be pa=5.16 bar, pb=5.04...
Questions



Mathematics, 22.07.2019 01:30

Mathematics, 22.07.2019 01:30


Chemistry, 22.07.2019 01:30

Mathematics, 22.07.2019 01:30


History, 22.07.2019 01:30

Physics, 22.07.2019 01:30


Advanced Placement (AP), 22.07.2019 01:30


History, 22.07.2019 01:30

Chemistry, 22.07.2019 01:30



Health, 22.07.2019 01:30

Mathematics, 22.07.2019 01:30

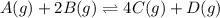
![K_p=\frac{[p_{D}]\times [p_{C}]}^4{[p_{B}]^2\times [p_{A}]}](/tpl/images/0346/8447/cda48.png)
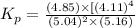
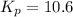
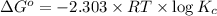
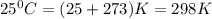
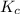 = equilibrium constant = 10.6
= equilibrium constant = 10.6