
Chemistry, 26.10.2019 00:43 gizmo50245
Aspirin (c9h8o4) is synthesized by reacting salicylic acid (c7h6o3) with acetic anhydride (c4h6o3) according to the reaction: c7h6o3 + c4h6o3 --> c9h8o4 + hc2h3o2 a. what mass of acetic anhydride is needed to completely react 1.00 x 10 4 g of salicylic acid?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2


Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
Aspirin (c9h8o4) is synthesized by reacting salicylic acid (c7h6o3) with acetic anhydride (c4h6o3) a...
Questions

Mathematics, 23.06.2019 06:00

English, 23.06.2019 06:00

Mathematics, 23.06.2019 06:00



Social Studies, 23.06.2019 06:00


Mathematics, 23.06.2019 06:00


Mathematics, 23.06.2019 06:00


Mathematics, 23.06.2019 06:00

Business, 23.06.2019 06:00

Mathematics, 23.06.2019 06:00




Mathematics, 23.06.2019 06:00


Mathematics, 23.06.2019 06:00

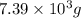 of acetic anhydride is needed
of acetic anhydride is needed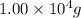 of salicylic acid =
of salicylic acid = 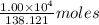 of salicylic acid
of salicylic acid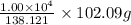 =
= 

