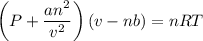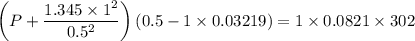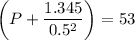Chemistry, 26.10.2019 01:43 meadowsoares7
If 1.00 mol of argon is placed in a 0.500-l container at 29.0 ∘c , what is the difference between the ideal pressure (as predicted by the ideal gas law) and the real pressure (as predicted by the van der waals equation)? for argon, a=1.345(l2⋅atm)/mol2 and b=0.03219l/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3


Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
If 1.00 mol of argon is placed in a 0.500-l container at 29.0 ∘c , what is the difference between th...
Questions

Mathematics, 07.04.2020 17:25


Mathematics, 07.04.2020 17:25





Health, 07.04.2020 17:25

Mathematics, 07.04.2020 17:25

Computers and Technology, 07.04.2020 17:25

Social Studies, 07.04.2020 17:25


Mathematics, 07.04.2020 17:25