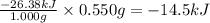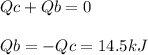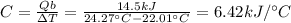Chemistry, 26.10.2019 02:43 holman9308
The combustion of exactly 1.000 g of benzoic acid in a bomb calorimeter releases 26.38 kj of heat. if the combustion of 0.550 g of benzoic acid causes the temperature of the calorimeter to increase from 22.01∘c to 24.27∘c, calculate the heat capacity of the calorimeter.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:50
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
The combustion of exactly 1.000 g of benzoic acid in a bomb calorimeter releases 26.38 kj of heat. i...
Questions

History, 06.11.2020 22:30

Mathematics, 06.11.2020 22:30

Biology, 06.11.2020 22:30



Mathematics, 06.11.2020 22:30


Mathematics, 06.11.2020 22:30

Arts, 06.11.2020 22:30


Mathematics, 06.11.2020 22:30





Social Studies, 06.11.2020 22:30

History, 06.11.2020 22:30


Mathematics, 06.11.2020 22:30

Mathematics, 06.11.2020 22:30