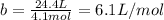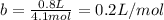Chemistry, 26.10.2019 03:43 barnhill6534
It turns out that the van dar waals constant b is equal to four times the total volume actually occupied by the molecules of a mole of gas. using this figure, calculate the fraction of the volume in a container actually occupied by ar atoms:
a) at stp
b) at 100 atm pressure and 0 degrees celsius

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1


Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1
You know the right answer?
It turns out that the van dar waals constant b is equal to four times the total volume actually occu...
Questions

Mathematics, 17.10.2019 02:00




Mathematics, 17.10.2019 02:00

Mathematics, 17.10.2019 02:00

Mathematics, 17.10.2019 02:00



Mathematics, 17.10.2019 02:00



Mathematics, 17.10.2019 02:00


Mathematics, 17.10.2019 02:00

Mathematics, 17.10.2019 02:00

Chemistry, 17.10.2019 02:00

Mathematics, 17.10.2019 02:00

Mathematics, 17.10.2019 02:00

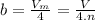 (1)
(1)