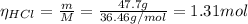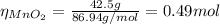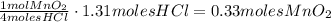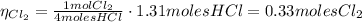Chemistry, 26.10.2019 04:43 jamaiciaw6
Chlorine gas can be prepared in the laboratory by the reaction of hydrochloric acid with manganese(iv) oxide:
4hcl(aq) + mno2(s) > mncl2(aq) + 2h2o(l) + cl2(g)
you add 42.5 g of mno2 to a solution containing 47.7 g of hcl.
(a) what is the limiting reactant? mno2 or hcl?
(b)what is the theortical yield of co2?
(c) if the yield of the reaction is 79.9%, what is the actual yield of chlorine?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
Chlorine gas can be prepared in the laboratory by the reaction of hydrochloric acid with manganese(i...
Questions

Social Studies, 20.07.2019 12:20



History, 20.07.2019 12:20

English, 20.07.2019 12:20

Mathematics, 20.07.2019 12:20


Chemistry, 20.07.2019 12:20

Biology, 20.07.2019 12:20

Chemistry, 20.07.2019 12:20

Social Studies, 20.07.2019 12:20

English, 20.07.2019 12:20

History, 20.07.2019 12:20

Mathematics, 20.07.2019 12:20




Mathematics, 20.07.2019 12:20

Business, 20.07.2019 12:20

History, 20.07.2019 12:20