
Chemistry, 26.10.2019 04:43 Halessoftball
What is the molar concentration of fe2+ ion in an aqueous solution if 37.50 ml of 0.118 m kbro3 is required for complete reaction with 11.00 ml of the fe2+ solution? the net ionic equation is: 6fe2+(aq)+bro3−(aq)+6h+(aq)→6fe3+(a q)+br−(aq)+3h2o(l).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

You know the right answer?
What is the molar concentration of fe2+ ion in an aqueous solution if 37.50 ml of 0.118 m kbro3 is r...
Questions

Mathematics, 05.07.2019 01:30

Biology, 05.07.2019 01:30

Mathematics, 05.07.2019 01:30


Social Studies, 05.07.2019 01:30


Physics, 05.07.2019 01:30

English, 05.07.2019 01:30





History, 05.07.2019 01:30

Mathematics, 05.07.2019 01:30

English, 05.07.2019 01:30

Mathematics, 05.07.2019 01:30


Mathematics, 05.07.2019 01:30

Social Studies, 05.07.2019 01:30

Mathematics, 05.07.2019 01:30

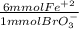 = 26.55 mmol Fe²⁺
= 26.55 mmol Fe²⁺

