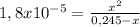Chemistry, 26.10.2019 05:43 blanca04fp
The degree to which a weak base dissociates is given by the base-ionization constant, kb. for the generic weak base, b b(aq)+h2o(l)⇌bh+(aq)+oh−(aq) this constant is given by kb=[bh+][oh−][b] strong bases will have a higher kb value. similarly, strong bases will have a higher percent ionization value. percent ionization=[oh−] equilibrium[b] initial×100% strong bases, for which kb is very large, ionize completely (100%). for weak bases, the percent ionization changes with concentration. the more dilute the solution, the greater the percent ionization. ammonia, nh3, is a weak base with a kb value of 1.8×10−5.parta what is the ph of a 0.245 m ammonia solution? partb what is the percent ionization of ammonia at this concentration?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

You know the right answer?
The degree to which a weak base dissociates is given by the base-ionization constant, kb. for the ge...
Questions

Mathematics, 24.03.2021 03:10

Mathematics, 24.03.2021 03:10


Health, 24.03.2021 03:10

History, 24.03.2021 03:10

Mathematics, 24.03.2021 03:10

Mathematics, 24.03.2021 03:10

Mathematics, 24.03.2021 03:10


Engineering, 24.03.2021 03:10

English, 24.03.2021 03:10

Mathematics, 24.03.2021 03:10



Mathematics, 24.03.2021 03:10



Mathematics, 24.03.2021 03:10


Mathematics, 24.03.2021 03:10