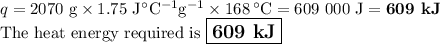Chemistry, 27.10.2019 01:43 bombbomb8449
The specific heat of a certain type of cooking oil is 1.75 j/(g⋅°c). how much heat energy is needed to raise the temperature of 2.07 kg of this oil from 23 °c to 191 °c?
from q to j

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 10:30
Can anyone explain 1. review your spectrometry data and use the known elements to identify the star's composition. which unknown elements make up this star? justify your element selections. 2. in parts i and ii of the lab, what happened to the electrons of each element to produce the different colors of light? explain your answers using important terms from the lesson and information provided in the laboratory. 3. stars composed of heavier (more massive) elements are often slightly older than stars made predominantly from hydrogen and helium. based on your data, is the newly discovered star a younger star? explain your answer.
Answers: 2
You know the right answer?
The specific heat of a certain type of cooking oil is 1.75 j/(g⋅°c). how much heat energy is needed...
Questions


Mathematics, 19.10.2020 05:01





English, 19.10.2020 05:01

Biology, 19.10.2020 05:01

Mathematics, 19.10.2020 05:01

Health, 19.10.2020 05:01

History, 19.10.2020 05:01

Chemistry, 19.10.2020 05:01

Spanish, 19.10.2020 05:01

Mathematics, 19.10.2020 05:01



Mathematics, 19.10.2020 05:01