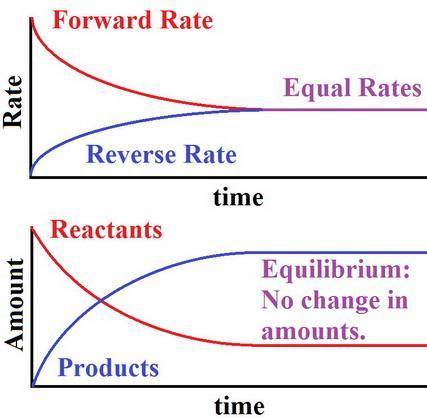
At chemical equilibrium, the amount of (product and reactant is zero, product and reactant remains constant, product is zero) because (all molecules have the same activation energy, an equal number of molecules take part in the reaction, the rates of the forward and reverse reactions are equal)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1
You know the right answer?
At chemical equilibrium, the amount of (product and reactant is zero, product and reactant remains c...
Questions


English, 03.05.2021 18:20

Mathematics, 03.05.2021 18:20


Mathematics, 03.05.2021 18:20









Mathematics, 03.05.2021 18:20

Mathematics, 03.05.2021 18:20



History, 03.05.2021 18:20

History, 03.05.2021 18:20

Biology, 03.05.2021 18:20