
Chemistry, 27.10.2019 02:43 Queenofpizza
Determine the mass in grams of 3.00 × 10²¹ atoms of arsenic. (the mass of one mole of arsenic is 74.92 g.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

You know the right answer?
Determine the mass in grams of 3.00 × 10²¹ atoms of arsenic. (the mass of one mole of arsenic is 74....
Questions

Business, 30.09.2021 18:20


Mathematics, 30.09.2021 18:20

History, 30.09.2021 18:20

Mathematics, 30.09.2021 18:20



Health, 30.09.2021 18:20



Chemistry, 30.09.2021 18:20

Business, 30.09.2021 18:20



Law, 30.09.2021 18:20

Business, 30.09.2021 18:20


Computers and Technology, 30.09.2021 18:20

History, 30.09.2021 18:20

History, 30.09.2021 18:20

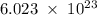 .
.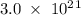 atoms.
atoms.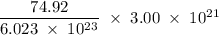 grams
grams


