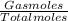Chemistry, 28.10.2019 18:31 katswindle11
Agaseous mixture containing 1.5 mol ar and 3.5 mol co2 has a total pressure of 8.6 atm. what is the partial pressure of co2?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
Agaseous mixture containing 1.5 mol ar and 3.5 mol co2 has a total pressure of 8.6 atm. what is the...
Questions



Mathematics, 30.11.2020 14:00

Mathematics, 30.11.2020 14:00

World Languages, 30.11.2020 14:00

Chemistry, 30.11.2020 14:00




Social Studies, 30.11.2020 14:00


History, 30.11.2020 14:00


English, 30.11.2020 14:00



Chemistry, 30.11.2020 14:00


Social Studies, 30.11.2020 14:00

Mathematics, 30.11.2020 14:00

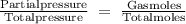
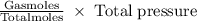
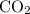 =
= 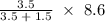
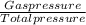 =
=