
Chemistry, 28.10.2019 21:31 altstattlana
Be sure to answer all parts. sulfuric acid is commonly used as an electrolyte in car batteries. suppose you spill some on your garage floor. before cleaning it up, you wisely decide to neutralize it with sodium bicarbonate (baking soda) from your kitchen. the reaction of sodium bicarbonate and sulfuric acid is na2co3(s) + h2so4(aq) → na2so4(aq) + 2co2(g) + 2h2o(l) you estimate that your acid spill contains about 5.1 mol h2so4. what mass of nahco3 do you need to neutralize the acid

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2


You know the right answer?
Be sure to answer all parts. sulfuric acid is commonly used as an electrolyte in car batteries. supp...
Questions





Mathematics, 26.02.2021 01:00




Chemistry, 26.02.2021 01:00

Health, 26.02.2021 01:00



Chemistry, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00

Biology, 26.02.2021 01:00





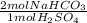 = 10.2 mol NaHCO₃
= 10.2 mol NaHCO₃

