Chemistry, 28.10.2019 23:31 devikapal101
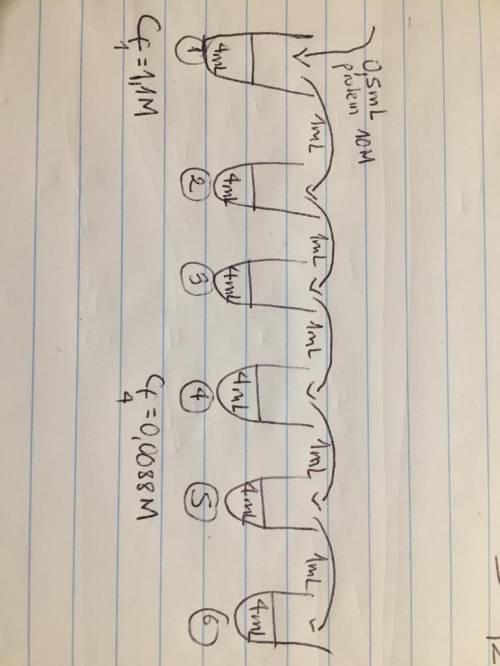
Serial dilution problem: six test tubes are placed in a rack. to each tube add 4 ml of saline solution. now to the first tube add 0.5 ml of concentrated protein (10 m) and mix well. then transfer 1 ml of tube # 1 to tube # 2 and mix well. 1 ml of the contents of tube # 2 is then transferred to tube # 3, and the procedure is repeated for the remaining tubes. what is the dilution factor and molar concentration of protein in tube # 4? show your answer by drawing 6 tubes and label them.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2
You know the right answer?
Serial dilution problem: six test tubes are placed in a rack. to each tube add 4 ml of saline solut...
Questions


Mathematics, 28.10.2020 18:10















Mathematics, 28.10.2020 18:10

History, 28.10.2020 18:10

Mathematics, 28.10.2020 18:10

Chemistry, 28.10.2020 18:10