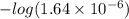Carbon dioxide dissolves in water to form carbonic acid, which is primarily dissolved co2. dissolved co2 satisfies the equilibrium equation co2(g) co2(aq) k=0.032 the acid dissociation constants listed in most standard reference texts for carbonic acid actually apply to dissolved co2. for a co2 partial pressure of 1.9x10-4 bar in the atmosphere, what is the ph of water in equilibrium with the atmosphere? (for carbonic acid ka1 = 4.46x10-7 and ka2 = 4.69x 10-11).

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
If 200.0g of copper(ll) sulfate react with an excess of zinc metal, what is the theoretical yield of copper
Answers: 1

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2
You know the right answer?
Carbon dioxide dissolves in water to form carbonic acid, which is primarily dissolved co2. dissolved...
Questions


Biology, 17.11.2020 22:30


Mathematics, 17.11.2020 22:30


Mathematics, 17.11.2020 22:30

English, 17.11.2020 22:30

Mathematics, 17.11.2020 22:30





Business, 17.11.2020 22:30


History, 17.11.2020 22:30



History, 17.11.2020 22:30

Mathematics, 17.11.2020 22:30

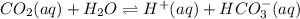
 dissolved as follows.
dissolved as follows.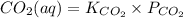
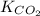 = 0.032 M/atm and
= 0.032 M/atm and 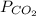 =
= 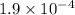 atm.
atm.![[CO_{2}]](/tpl/images/0350/3284/0a7e9.png) will be calculated as follows.
will be calculated as follows.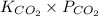
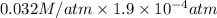
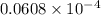
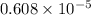
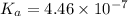
![K_{a} = \frac{[H^{+}]^{2}}{[CO_{2}]}](/tpl/images/0350/3284/deb36.png)
![4.46 \times 10^{-7} = \frac{[H^{+}]^{2}}{0.608 \times 10^{-5}}](/tpl/images/0350/3284/7b4bd.png)
![[H^{+}]^{2}](/tpl/images/0350/3284/724e3.png) =
= 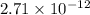
![[H^{+}]](/tpl/images/0350/3284/85507.png) =
= 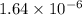
![-log [H^{+}]](/tpl/images/0350/3284/822be.png)