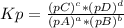Chemistry, 29.10.2019 02:31 Diegosolorzano50
The following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10-3calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g)write the equilibrium constant expression, kp write the pressures in the following format: (pco2)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1
You know the right answer?
The following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1...
Questions









History, 10.07.2019 20:00

History, 10.07.2019 20:00



Physics, 10.07.2019 20:00

Mathematics, 10.07.2019 20:00

Biology, 10.07.2019 20:00


Social Studies, 10.07.2019 20:00

Social Studies, 10.07.2019 20:00

Mathematics, 10.07.2019 20:00

Advanced Placement (AP), 10.07.2019 20:00