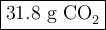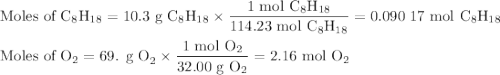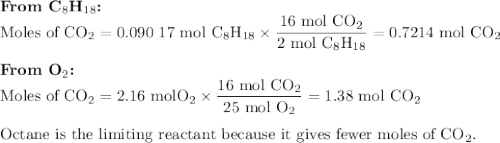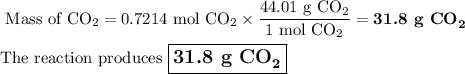Chemistry, 29.10.2019 04:31 iamabouttofail
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 10.3 g of octane is mixed with 69. g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to significant digits.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1
You know the right answer?
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . s...
Questions


Social Studies, 04.02.2021 22:40




Mathematics, 04.02.2021 22:50

Mathematics, 04.02.2021 22:50

Biology, 04.02.2021 22:50

Mathematics, 04.02.2021 22:50


Biology, 04.02.2021 22:50

Spanish, 04.02.2021 22:50

History, 04.02.2021 22:50

Mathematics, 04.02.2021 22:50

Business, 04.02.2021 22:50

English, 04.02.2021 22:50

Health, 04.02.2021 22:50

Mathematics, 04.02.2021 22:50

Health, 04.02.2021 22:50